Abstract
Background:
Voxelotor (Oxbryta®) is a small molecule that binds to the alpha chain of hemoglobin (Hb) and increases the affinity of Hb for oxygen which reduces sickle Hb polymerization. It was approved by the FDA in 2019 for the treatment of sickle cell disease (SCD). Currently, the only method available to estimate the concentration of voxelotor in the blood is to obtain exposure measurements which are only available in select research laboratories. A method to measure voxelotor at a standard laboratory would allow clinicians to assess compliance and may be useful in determining optimal dosing. Case studies have reported that voxelotor binding to Hb interferes with capillary zone electrophoresis (CZE). As previously reported, in CZE the characteristic peaks of hemoglobin A2 (HbA2) and hemoglobin F (HbF) split in the presence of voxelotor. Interestingly, it does not cause the hemoglobin S peak to split. We posited that we could use this split to estimate the presence of voxelotor and whole blood concentration. Biophysical measurements of voxelotor binding to Hb were also measured in these samples.
Methods:
Patients were enrolled prospectively in an IRB approved protocol. Voxelotor was initiated at 1500 mg daily on day 0 and samples were taken at day 0 (pre-dose), 14, 30, and 60. Samples had Hb variants quantified by CZE using the Capillarys 2 FlexPiercing Instrument (Sebia, Georgia). Hematology parameters were measured with the Sysmex XN-1000 automated analyzer (Sysmex, Illinois).
To determine whole blood concentration of voxelotor, samples were sent to Worldwide Clinical Trials where a validated liquid chromatography-tandem mass spectrometry method was used.
Voxelotor's interference with HbA2 on CZE is dependent on the HbF percentage, therefore samples from patients with SCD were combined into three pooled samples (5-10 samples per pool) of HbF percentages spanning 5-30%. Three hundred µL aliquots of each pool were spiked with voxelotor in DMSO in triplicate to different concentrations between 0 and 600 µMol/L and were incubated at room temperature for 1 hour before being tested with CZE. Samples were then analyzed for voxelotor interference resulting in split peaks of HbF and HbA2. HbA2 interference percent (%VarA2) was calculated as the reported value of the HbA2 split peak over the total HbA2 value (both split and parent peak) times 100. F% was used as directly reported by the instrument without consideration of voxelotor interference. Results were then analyzed in Excel (Data Analytics package) using a multiparameter regression to generate a line of best fit. To allow for logarithmic fit when examining the correlation of calculated concentration with increase in Hb due to voxelotor, samples with negative Hb rises were excluded and concentrations which resulted as negative values were changed to 0.01 µM.
Results:
Of 20 patients which have been enrolled to date, 9 patients have completed the study and their data was used for these analyses. Using the CZE method described above the concentration of voxelotor was quantifiable using the following equation.
Equation 1: uM voxelotor = -99.13 + 7.10*%HbF +12.52*%VarA2
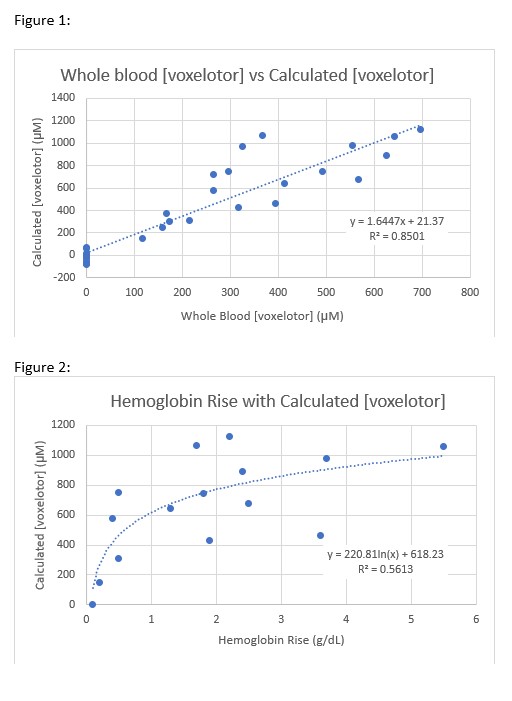
The calculated concentrations of voxelotor based on CZE results had a strong correlation with whole blood concentration (R 2 = 0.85, p <0.001). (Figure 1)
When calculated concentration was compared to change in Hb at days 14, 30, and 60 there was a significant positive logarithmic correlation between concentration and change in Hb (R 2=.56, p<0.01). (Figure 2)
Conclusions:
Using equation 1, CZE can be used to detect the presence of voxelotor and estimate its whole blood concentration. This will allow clinicians to have a better understanding of how their patients are using voxelotor. Additionally, higher calculated whole blood concentrations correlated with higher increases in Hb. It was previously shown that patients who receive higher doses of voxelotor have on average larger increases in Hb. If it could be shown that increasing concentration in an individual on voxelotor is associated with an increased Hb for that individual, then our method could also be used to help clinicians select and adjust doses of voxelotor in a similar manner to how HbF is used in hydroxyurea dosing.
Curtis: GBT: Consultancy. Minniti: CSL Behring: Other: Endpoint adjudicator; Bluebird Bio: Other: Endpoint adjudicator; F. Hoffmann-La Roche: Consultancy; Chiesi: Consultancy; Novo Nordisk: Consultancy; Forma: Consultancy; Novartis: Consultancy; GBT: Consultancy. Ngyuen Dang: GBT: Current Employment. Pochron: GBT: Current Employment. Campbell: GBT: Research Funding; Sebia: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal